Please read and ask your elected officials to expose the climate change fraud. Thanks. Adrian Arp. Remember as President Washington said in 1794, “Truth will ultimately prevail where the pains is taken to bring it to light.”
Greetings Senators Crapo and Risch; Representatives Simpson and Fulcher; Governor Little; Lt. Governor Bedke and the Idaho State Legislature:
As a scientist, I found the following analysis much more believable that the climate alarmists scare tactic that the earth will be destroyed by global warming/climate change in a few years. Of course, there is NO credible science that the earth will be destroyed by the very small amount of Carbon Dioxide in the atmosphere (only 415 PPM). Our plants would like 1500 to 2000 PPM.
The globalists are using the global climate change HOAX to control all human activity on the planet, destroy our economy and redistribute our wealth. This must be exposed and stopped! When will one of you finally take your oath of office seriously to expose the unscientific HOAX that CO2 is killing the planet? CO2 is NOT a pollutant! Without CO2 we will have NO food to eat or oxygen to breathe.
A concerned citizen, Adrian Arp, Ph.D. Please take time to read this interesting article. I would appreciate your comments and hopefully expose the HOAX!
The End of Life on Earth in about nine million years
By VAN SNYDER
The fake end of the world: We all know that the anti-industry industry wants to eliminate CO2 emissions that result from producing energy. Ever since they switched gears from *The Coming Ice Age!* to *The Runaway Greenhouse!* they’ve told us that increasing CO2 in the atmosphere is a bad thing that threatens the planet. They’ve managed to bake that into the social discourse, so firmly that contradicting them can get you canceled on Instagram or Facebook, or maybe even on Twitter, and get you fired from your job.
What’s their argument? They insist that if the concentration of CO2 in the atmosphere increases from today’s 415 parts per million by volume (ppm), the temperature will increase by two degrees Celsius — about the difference between the average temperatures in New York and Miami — and that this would be harmful.
They say this with a straight face, even though one of their high priests, Stephen Schneider, wrote in 1971 that EACH doubling/of CO2 concentration in the atmosphere would ADD/0.81 degrees Celsius to the Earth’s average temperature, and even increasing CO2 concentration by a factor of ten would increase temperature by only 2.5 degrees Celsius. His conclusion was that, as much as we might hope to be able to do it, no matter how much coal and petroleum and natural gas we burned, we could not prevent the coming ice age. How can the same guy who brought us that message now claim that we’re on the verge of a runaway greenhouse?
When you multiply one phenomenon by a fixed amount, and the phenomenon it causes endures an addition, not a multiplication, by a fixed amount, the two are said to be in a logarithmic relationship. Svante Arrhenius noticed this about the atmosphere in 1908 — and Stephen Schneider repeated it in 1971.
They’ve also warned us that rising sea levels will soon inundate Bangladesh. But the Ganges Delta, which includes pretty much all of Bangladesh, is silting up faster than two millimeters per year, which is the rate of sea level increase about which our betters complain.
They also warn us that coral atolls will be submerged and die, as if they cannot cope with sea level rise. We know they’re able to cope with sea level rising much faster than two millimeters per year. How do we know that? At the depth of the ice age, about 8,000 years ago, sea levels were 400 feet lower than they are now. That means, on average, sea level increased 15 millimeters per year. The ice that covered Montreal to a depth of 3 kilometers hasn’t been melting at a uniform rate for 8,000 years. It melted much faster a long time ago, and less rapidly recently. So at the end of the ice age, sea levels rose much faster than 15 millimeters per year.
But coral didn’t evolve at some time long after the end of the ice age, after sea level stopped rising in the centimeters-per-year range. It’s been around for at least 25 million years. How is it still here?
How can it be that sea levels rising at the rate of centimeters or tens of centimeters per year didn’t spell corals’ doom, but sea levels rising at the rate of two millimeters per year will?
Well, coral can obviously deal with increasing sea levels. But climate activists cannot. If they had beachfront property, they would stand in the ocean, as its level crept up past their ankles to their knees to their necks at the rate of two millimeters per year, without moving, complaining that you’re not driving a Tesla, while they drowned. Maybe they would do that, but even the lowest creatures wouldn’t.
Then climate activists will tell us that the oceans are becoming more acidic because when CO2 dissolves in fresh water, it becomes more acidic, which will dissolve seashells and coral reefs and fishes bones and the coccoliths of phytoplankton. There are at least two problems with that argument. One is that shellfish live quite happily, without their shells dissolving, in acidic river estuaries. It is clear they have biological mechanisms, mostly using mucus, to control acidity where they’re depositing limestone crystals.
The other problem with the argument is that adding CO2 to seawater has a very different effect from adding it to fresh water. When a weak acid is added to a strong base (alkaline material), or when a weak base is added to a strong acid, the result is what chemists call a/buffer solution/. The acidity (or alkalinity) changes very little. Much less than it would in pure water. Oceans contain enormous amounts of sodium bicarbonate, more commonly called baking soda. It’s a strong base, but not as strong a base as the lye that is the primary ingredient of Drano. When CO2 dissolves in seawater, the acidity of the oceans changes very little.
The real end of the world:
At the end of the Jurassic period, about 150 million years ago, the CO2 concentration in the atmosphere was about 2,500 ppm. It’s been declining since then on an almost straight path.
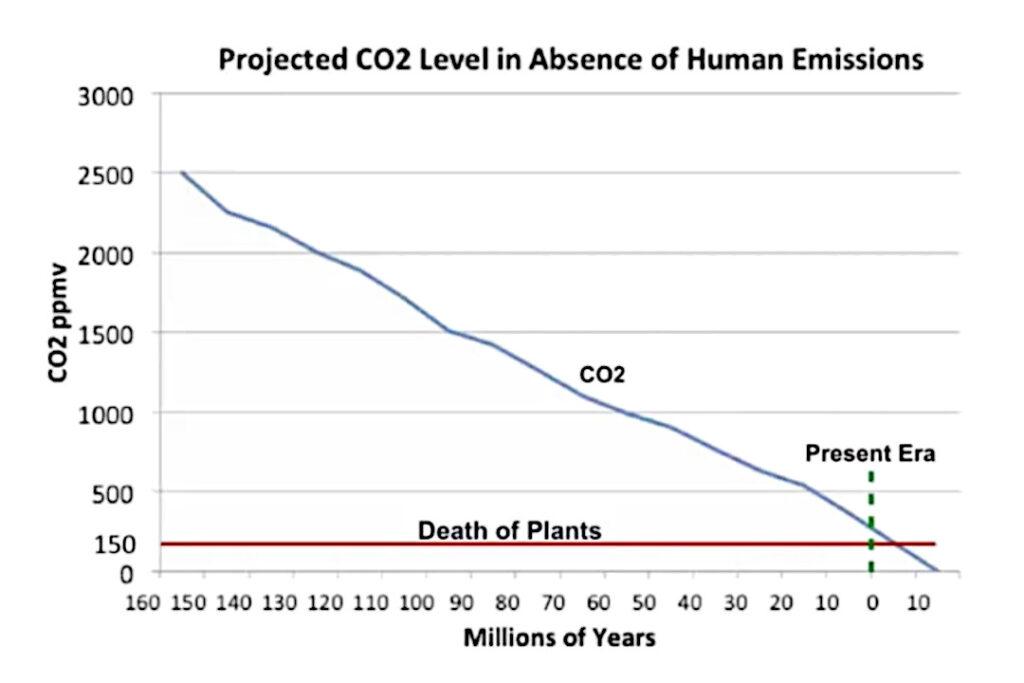
This would probably make the climate alarmists very happy. The CO2 concentration in the atmosphere was reduced to about 280 ppm in 1750. How did this happen? Did dinosaurs stop burning coal and driving SUVs?
About 500 million years ago, sea creatures struggled with unwanted crystallization of calcium carbonate, better known as limestone or chalk. It harmed their bodies, which were largely jelly. But they eventually learned to control it, and started using it to armor plate themselves against predators. Clams and snails made shells. Coral made castles. Phytoplankton made elaborately beautiful structures called coccoliths that are generally too small to appreciate without a microscope.
When these creatures die, their limestone structures don’t disappear. They sink to the bottom of the sea, where enormously thick beds of limestone and chalk accumulate, sequestering carbon dioxide almost forever. Some of it is subducted under the continents, and is then decomposed into calcium oxide and carbon dioxide by volcanoes. But most of it is gone forever. Every continental area that was once a seabed, such as the American midwest, places like Indiana, have limestone deposits that are hundreds of feet thick.
Climate alarmists are cheering, but if you’re not a climate alarmist, you should be at least mildly alarmed, rather than cheering. Why?
Because plants need CO2. When the CO2 concentration in the atmosphere falls below about 150 ppm, plants die. When plants die, the only forms of life that remain are bacteria and viruses, and maybe fungi. When the CO2 concentration in the atmosphere falls below about 150 ppmv, it really is the end of the world.
If you calculate the slope of the line from a CO2 concentration of 2,500 ppm about 150 million years ago, to 280 ppm in 1750, the result is that the CO2 concentration had been decreasing at the rate of about (2,500 – 280) / 150 = 2220 / 150 = 14.8 ppm per million years. How long would it have taken to decrease from 280 ppm to 150 ppm? That’s easy: (280 – 150) / 14.8 = 130 / 14.8 = 8.8 million years. That’s when the end of the world would have arrived if nothing had happened in 1750.
Fortunately, the industrial revolution began, and we started burning coal and hydrocarbons, which increased the in the atmosphere to 415 ppm. If we stop emitting CO_2 , when will the Earth die? that’s another easy calculation: (415 – 150) / 14.8 = 265 / 14.8 = 17.9 million years.
Instead of wringing your hands about increasing carbon dioxide in the atmosphere, you should be cheering. Those evil coal mines and oil wells have postponed the end of the world by about nine million years.
We really ought to do a lot more.
It is a well-known fact that plants grow better if the CO2 concentration is greater, within reasonable limits. This isn’t some vague hand-waving conjecture. Agronomists have measured it. They’ve not just measured it in greenhouses, but in open fields. Commercial greenhouse operators buy CO or they burn kerosene or gasoline or propane or butane to increase the concentration of CO2 in their greenhouses to the range from 800 to 2,000 ppm, depending upon what they’re growing (and how much CO2 they can afford to buy or make).
Increasing the CO2 concentration in the atmosphere to that range would increase forests, and more importantly for an expanding population, it would increase food production.
After the Cambrian explosion about 500 million years ago, plants developed the ability to make lignin, the primary structural component in wood. But it was another hundred million years before fungi developed the enzyme lignase, which enables them to metabolize lignin. The result was that forests piled atop one another for a hundred million years, eventually compressing their dead ancestors into coal seams hundreds of feet thick. Essentially all of the coal was laid down in the Carboniferous and Permian Periods, continuing more slowly during the Permian Period because fungi were starting to metabolize lignin. At its depth, the CO2 concentration in the atmosphere was 180 ppmv, only 30 ppmv above the level at which higher forms of life would have ceased to exist on the Earth. So we were saved by fungi, and then to a significant extent by volcanism in the Triassic and Jurassic Periods, which decomposed subducted limestone into calcium oxide and CO2. But volcanism has declined significantly since the end of the Jurassic period, and so the CO2 concentration in the atmosphere has been steadily declining.
It is an open question whether human civilization will last for 17.9 million more years, but even today, humans could help to preserve life on Earth for quite a bit longer than that by burning coal as fast as we can (cleanly of course), and making cement (that is, decomposing limestone into calcium oxide and carbon dioxide) as fast as we can. It’s the least we could do for our distant descendants. After all, they’ll still be crushed paying off the Biden Administration’s debt.
The climate alarmists have it all wrong.



2 replies on “The Fake End of the World”
Is this knowledge taught in Idaho dot gov schools? The truth sets you free. The climate hoax is the prison we are now kept within.
Any middle school student should be able to tell you that this is a hoax. But why can’t they say that? Because the basic science classes being taught our students have failed! Students can’t put 1+2 = 3 anymore. Students are not taught to THINK. Co2 plus PLANTS equals O2.